Fractionation of Ge/Si ratios in clays
Fractionation of Ge/Si ratios in clays, how to move forward to use them in geochemical codes
Ge/Si ratios are useful weathering tracers. So far, we have been using Ge/Si ratios empirically, based on global estimates of Ge/Si in rivers and soils. A thermodynamic basis to the partitioning behavior of Ge into clays is necessary to incorporate Ge/Si ratios into geochemical modeling codes — such as a CrunchFlow, Phreeqc or GWB. These codes use the solubility of minerals. However, until today there is no thermodynamic data for alumino-germanate clays — although, there is some thermo data for some phyllo-germanates (Shtenberg et al. 2017).
For Ge-bearing clays forming a solid-solution with Si, the fractionation factor depends on the stochiometry of the clay.
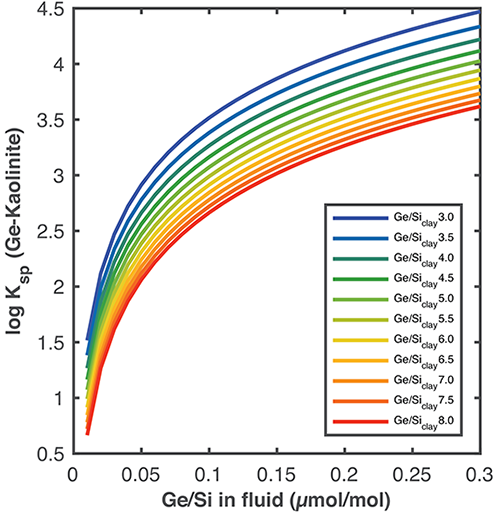
Solubility constants as a function of Ge/Si ratios in the fluid. The colored curves show the trajectories for given (Ge/Si)clay ratios.
Transient behavior of trace element geochemical tracers
In natural systems several factors can influence how trace elements are partitioned into minerals. Fluid chemistry and dissolution/precipitation rates can affect Ge/Si ratios at short timescales. Although we don’t have a complete understanding of the precipitation dynamics of secondary clays in the Critical Zone, we can couple geochemical tracers into RTMs to test for different scenarios. Using CrunchFlow’s isotope block, we can model the fractionation of isotope and trace element tracers — such as Ge/Si ratios — and test for factors influencing precipitation mechanisms.
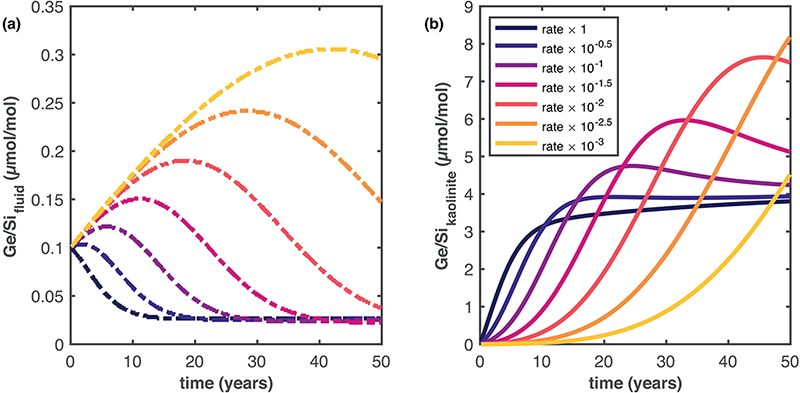
(a) The effect of a slower precipitation rate constants for kaolinite in the evolution of Ge/Si ratios in the fluid (dash-dot lines). The control case (perfect fractionation) is shown in blue and slower rate constants for a linear TST rate law in 0.5 log-units reductions. (b) Evolution of Ge/Si ratios in the precipitated mineral solid solution (solid lines), color symbology is the same as in (a).
