Sources and long-term accumulation of salt deposits in the Atacama Desert
Sources and long-term accumulation of salt deposits in the Atacama Desert
The giant nitrate deposits of the hyperarid Atacama Desert in Chile are one of the most extraordinary, yet enigmatic, mineral occurrences on Earth. These deposits are complex assemblages of highly soluble nitrates, chlorides, sulfates, perchlorates, iodates, and chromates, and their preservation is the result of prevalent hyperarid climate conditions in the Atacama Desert. Different isotopic tracers can help us decipher the sources and sinks of rare elements that accumulate in hyperarid and Mars-analog environments.
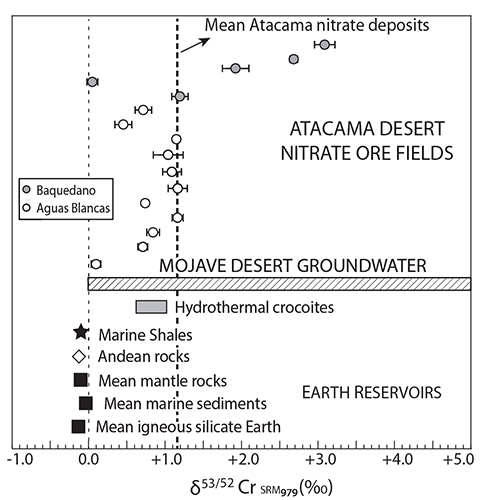
We used cosmogenic iodine (129I) and chromium isotope (δ53/52Cr) data of nitrates to demonstrate that groundwater played an unforeseen role in the formation of these massive deposits.
δ53/52Cr of nitrate samples from Atacama Desert (northern Chile). White and gray circles represent Aguas Blancas and Baquedano samples, respectively. Error bars are shown for each value. Mojave Desert (western USA) groundwater δ53/52Cr values are displayed in hachured rectangle (Izbicki et al., 2008, 2012). Black star and white diamond show δ53/52Cr value of Jurassic marine shales and igneous rocks from Atacama, respectively. Mean values for igneous silicate Earth, marine sediments, mantle rocks, and hydrothermal crocoites are shown for comparison (Schoenberg et al., 2008). Thick vertical dashed line shows average δ53/52Cr value for nitrates (+1.104‰), and light dashed line represents 0.0‰ value.
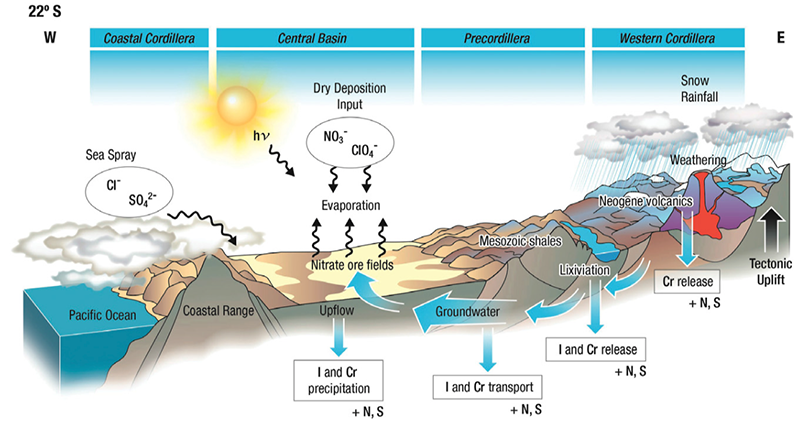
A conceptual multi-source genetic model for the formation of Atacama Desert’s giant nitrate deposits. Processes involved in formation of nitrate deposits are shown, i.e., atmospheric dry deposition, groundwater transport and precipitation, seaspray inputs, and evaporation. Iodine as iodate (IO3−) or iodide (I−); Chromium as chromate (CrO42−), dichromate (Cr2O72−), or Cr(III); Sulfur as sulfate (SO42−); Nitrogen as nitrate (NO3−). Perchlorate (ClO4−) and chloride (Cl−) are also shown.
